THE EXCHANGE | Newsletter - August 2023
Medanta Hospital Lucknow Launches Comprehensive Cancer Care Programme with North India’s First Varian Edge Machine
Medanta Hospital Lucknow launched its Comprehensive Cancer Care Programme with the inauguration of North India’s first Varian Edge radiation machine and a Chemotherapy Daycare Centre by Honourable Chief Minister of Uttar Pradesh Shri Yogi Adityanath in the presence of Mr.Pankaj Sahni (Group CEO, Medanta), Dr. Rakesh Kapoor (Medical Director, Medanta Hospital, Lucknow) and renowned cancer specialists from Medanta Gurugram. Dr. Naresh Trehan (Chairman and Managing Director, Medanta) addressed the gathering remotely, extending his best wishes on creating a world-class facility in the state.
The Comprehensive Cancer Care Programme aims to revolutionise cancer treatment in Uttar Pradesh with the use of cutting-edge technologies such as the Varian Edge, which allows doctors to plan and give radiation therapy with submillimetric precision.
The Programme follows Medanta’s holistic Tumour Board approach, which brings together doctors from all specialities to systematically assess each patient and curate an individualised, evidence-based treatment plan, in addition to offering round-the-clock specialised care for critically ill patients.
It has state-of-the-art infrastructure and is backed by one of the largest teams of cancer doctors in the state, including highly specialised and dedicated teams, comprising site-specific cancer surgeons, medical oncologists, radiation oncologists, haemato-oncologists, pathologists, radiologists, interventional radiologists, and oncology nurses committed to treating all types of cancer.
This facility is the first in Uttar Pradesh, excluding parts covered under the National Capital Region, to offer Total Body Irradiation needed for bone marrow transplant patients. Other advanced therapies offered here include stereotactic radiosurgery (SRS), stereotactic radiotherapy (SRT), and RapidArc Volumetric Arc Therapy.


“The Comprehensive Cancer Care Programme at Medanta - Lucknow brings under one roof comprehensive, compassionate cancer care of global standard for the people of Uttar Pradesh and surrounding states. The facility has cutting-edge technology like the Varian Edge, which allows our experienced doctors to plan and give radiation therapy with submillimetric precision. Backed by multi-superspecialist doctors, modern infrastructure, and advanced imaging and diagnostic tools, the programme creates an ideal ecosystem on par with the best in India. Patients and caregivers will no longer have to travel long distances for specialised second opinion or holistic cancer treatment.”
Dr. Naresh Trehan
Chairman and
Managing Director
Medanta
Infocus
Limb Salvage Surgery with Megaprosthesis in a Young Adult with Osteosarcoma
Osteosarcoma is a relatively rare primary malignant tumour of the bone with an estimated global incidence of 3.4 cases per million population. Although osteosarcoma is rare in the overall population, it is the most common bone cancer in children and young adults. Conventional osteosarcoma comprises about 80% of all osteosarcoma cases and usually affects young adults with peak incidence age of 10-30 years; it is more common in men than in women.
The past century has seen an improvement in survival rates with life expectancy rising from a mere 20% in the early part of the 20th century to about 65% in the recent years. The current management of osteosarcoma in early stages of the disease focuses on chemotherapy and limb salvage.
Case Study
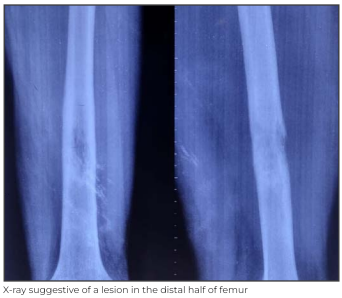
A 20-year-old male presented to Medanta - Lucknow with complaints of swelling in distal part of the left thigh over the past 1 month. Examination revealed tenderness; overlying skin was normal in appearance and touch. Neurological examination was normal without any signs of muscle wasting; there was normal range of movement at the knee joint.
Patient underwent radiography, which was suggestive of conventional osteosarcoma. This test was followed by a biopsy and histopathological examination of the lesion that confirmed the diagnosis.
The case was discussed and the patient was planned for neo-adjuvant therapy to shrink the lesion before doing the limb salvage surgery and then adjuvant chemotherapy.
After counselling and giving his consent, the patient underwent a limb salvage surgery post completion of neo-adjuvant therapy. The goal of surgical management of osteosarcoma is complete excision of the lesion, which typically involves resection with wide margins. This is achieved in two steps – initial resection and subsequent reconstruction.
A local wide excision of the tumour margin was done using the extensile para patellar approach incorporating the biopsy tract; there was no involvement of the neurovascular bundle. Excision was followed by metallic reconstruction using distal femur megaprosthesis.
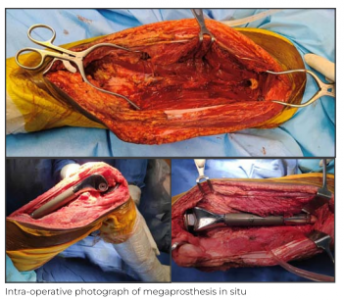
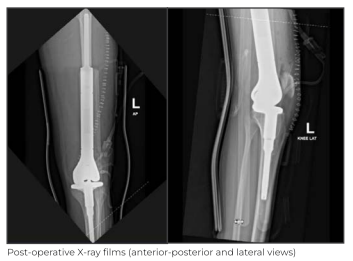
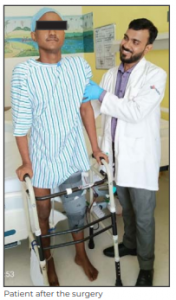
The surgery was successful with good clinical outcome. On Day 2 after the surgery, the patient was made to stand with support and was able to achieve assisted flexion of
90 degrees at the knee joint. The patient was discharged on Day 5 of the surgery and is being followed up in the OPD. The adjuvant chemotherapy session will be started soon after sutures are removed on post operative Day 15.
Discussion
As osteosarcoma is the most common primary osseous malignancy in the paediatric population, surgery presents a unique set of challenges. To achieve clear margins, excision may necessitate physeal resection that may cause growth disturbances as the child matures. In the past, a tumour that traversed the growth plate was considered to be an indication for amputation because there was no available means to restore function. With the advent of options that “grow” or expand with the patient, masses that cross the growth plate are no longer considered a contraindication to limb salvage.
Management of osteosarcoma requires a multidisciplinary team. Patients with suspected or confirmed osteosarcoma should be evaluated and treated at a comprehensive cancer care centre with a multidisciplinary sarcoma programme that includes paediatric, medical and radiation oncologists, orthopaedic and surgical oncologists, musculoskeletal pathologists, and radiologists. Successful treatment involves proper diagnosis, neoadjuvant and adjuvant multi-agent chemotherapy, and aggressive surgery with an emphasis on limb-preserving procedures.
The comprehensive cancer care programme at Medanta - Lucknow is backed by one of the largest teams of cancer doctors in the state. It is equipped to deliver advanced cancer treatments using the very best and latest in technology.
Dr. Saif N Shah
Director - Orthopaedics
Institute of Musculoskeletal Disorders and Orthopaedics
Medanta – Lucknow
Dr. Hatif Q. Siddiqui
Consultant - Orthopaedics
Institute of Musculoskeletal Disorders and Orthopaedics
Medanta - Lucknow
Medanta@Work
Lung Parenchymal Abnormalities and Outcomes in Hospitalised Patients with COVID-19 Pneumonia
Pulmonary fibrosis is one of the commonly reported sequelae with varying frequencies among the survivors of COVID-19. While cases have been reported of parenchymal lung abnormalities (PLA) on high-resolution computed tomography (HRCT) scan in patients with COVID-19 pneumonia with varying periods of follow-up, pulmonary consequences are clinically significant for potential therapeutic intervention and is highlighted as a key research priority.
To better understand the evolution of the patterns of PLA on HRCT over 12 weeks, we undertook a prospective observational study in adult patients of COVID-19 whose infection was confirmed via an RT-PCR test, and were hospitalised from June 2020 to September 2020 at Medanta - Gurugram.
Consecutive, consenting patients with acute symptoms of COVID-19 and no pre-existing awareness of PLA – both confirmed with an RT-PCR test and HRCT, respectively – were included in the study. Patients with clinical and/or radiological features suggestive of an alternative diagnosis were excluded. The supportive care and treatment regimen for individual patients were in accordance with the standard of care at the time.
A non-contrast, supine, inspiratory HRCT of the lungs was done at admission (CT1); it was repeated 4-8 weeks after admission (CT2), and then again at 10-12 weeks (CT3) in a subgroup of patients with persisting symptoms. Interpretation of HRCT image pattern was done by a team of 3 experienced chest radiologists blinded to the clinical severity and each other’s interpretation.
The distribution and patterns of PLA were pre-specified and agreed upon by 2 senior pulmonologists and chest radiologists. The PLA patterns and distribution were visualised at the same time as CT1, CT2, CT3 and the findings were interpreted without discussions among the radiologists. The final diagnosis was based on a multidisciplinary discussion with pulmonologists and the COVID-19 team in Medanta - Gurugram. Patients were followed up over telephone at 6 months post discharge to enquire about overall status of well-being using a structured questionnaire. Temporal changes in clinical severity, PLA and extent of lung involvement across CT1, CT2 and CT3 were presented and compared using the Chi-Square Test.
The study was approved by the Institutional Review Board and Ethics committee at
Medanta - Gurugram. Of the 179 patients who met the inclusion criteria, 145 were male (81%) with a median ± interquartile range (IQR) age of 57.9 years ± 9.7 years. There was pre-existing hypertension in 78 (44%) and diabetes in 72 (40%). At presentation, cough in 111 patients (63%) and fever in 153 (85%) were the predominant symptoms, with 15 (8%) complaining of dyspnea and 74 (41%) had a hospital stay of more than 10 days. Only one patient had pre-existing interstitial lung disease (ILD) while 17 patients had pre-existing chronic obstructive pulmonary disease (COPD) or asthma.
At presentation, 88 (44.7%) were on low-flow oxygen (<4L/min), 60 (33.5%) were on high-flow oxygen (>8L/min) and 7 (3.9%) on invasive ventilation. At 4-8 weeks, none of the patients needed to be on high-flow oxygen or ventilator support.
At baseline, the predominant feature was ground glass opacities (GGOs) in 144 (80.4%) patients; consolidation in 23 (12.9%) and reticulation in 7 (4%). At 4-8 weeks, there was a reduction in the number of GGOs to 123 (68.7%), consolidation to 10 (5.6%) and an increase in reticulation to 23 (12.9%). Of the 44 patients who returned for follow-up at 10-12 weeks due to persisting symptoms, GGOs were seen in 26 (59%) and reticulations were seen in 9 (20.5%) patients.
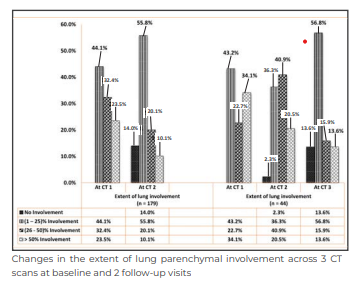
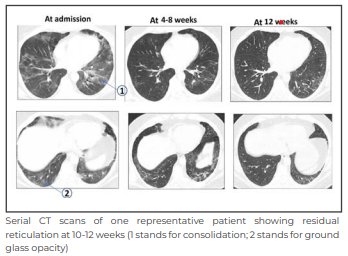
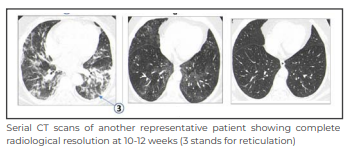
The above image shows the extent of lung involvement across 3 serial CT scans. The extent of lung involvement showed a substantial reduction over time in the 3 sets of HRCTs (CT1 vs CT2 p < 0.005; CT2 vs CT3 p < 0.0001; CT1 vs CT3 p < 0.008).
Our observations of the GGOs as the most common PLA, followed by consolidation at baseline is similar to the report of a systematic review of 109 reports involving 2,908 adults with COVID-19 that showed GGOs in 68% followed by consolidation in 48% of cases.
At 4-8 weeks, our study showed patterns of fibrosis in HRCT images of 12% cases. At 10-12 weeks (CT3), fibrosis was seen in 9 out of the sub-group of 44 patients. We interpret the latter finding with caution as CT3 was obtained in the sub-group of patients prompted by persistent respiratory symptoms and clinical decision of the treating physician.
Nevertheless, it is apparent that at least 5% (9 out of 179) patients hospitalised with COVID-19 pneumonia had HRCT evidence of pulmonary fibrosis at 3 months. Two recent meta-analyses have reported higher prevalence of fibrotic sequelae of 29% (95% CI: 22%–37%) and 45% (range: 9.3-84%) with a median follow-up of 3 months.
Our factual observations at 4-8 weeks, interpreted prospectively and precisely based on pre-specified criteria are in keeping with the interim analyses of the UK Interstitial Lung Disease (UKILD) Consortium-Long COVID study that estimated residual lung abnormalities in up to 11% people following COVID-related hospitalisation. In essence, our study confirms that our findings are complementary to the estimate from the
UKILD study.
A total of 144 out of 176 survivors who responded over the telephone at the 6-month follow-up, reported good overall sense of well-being and return to their pre-COVID health status. Studies from other cohorts have reported exertional dyspnea in more than 1/3rd of hospitalised COVID-19 patients at 12 months. Another large prospective multi-centric study (post-hospitalisation COVID-19 study) found that only 29% were fully recovered at 6 months. While these differences could be due to varying ethnic backgrounds, clinical severity, management protocols etc., we believe our results from patients’ cohort of Caucasoid origin, born and raised in India, not in Europe, are useful to understand the sequelae and consequences of COVID-19 pneumonia globally.
Limitations of our prospective observational study include findings in hospitalised patients at a single tertiary care hospital, shorter duration of follow-up, lack of pulmonary function tests to correlate with the PLA, 6-month follow-up was limited to phone calls in 144 out of the 176 survivors who responded, and third HRCT was obtained in the subgroup of 44 patients with persisting respiratory symptoms at 10-12 weeks. Acknowledging the challenges and difficulties associated with obtaining lung function test during the peak of the COVID-19 pandemic, our observations of PLA are a prospective study of a relatively large cohort of hospitalised patients with COVID-19 in the pre-vaccination era.
In summary, our study documents resolving or completely resolved PLA in 88% of 179 hospitalised patients with COVID-19, fine reticulations indicative of pulmonary fibrosis in 12% at 4-8 weeks, and fibrotic sequelae in at least 5% of patients at 10-12 weeks from baseline. At 6 months, 144 of the 176 survivors, who responded to follow-up phone calls, had returned to their normal pre-COVID health status. Contrary to most studies that have reported a significantly higher prevalence (30%-45%) of fibrotic sequelae post COVID-19 pneumonia, our findings of much lower prevalence are reassuring and send a positive message to the medical fraternity.
Results from our study and lessons from other studies related to COVID-19 pneumonia during this pandemic warrant consideration of prospective studies proactively to determine the clinical significance of long-term PLA, lung function impairment and the potential role of anti-fibrotics among those who manifest persistent pulmonary fibrosis after new variants of COVID-19 or other similar viral pneumonia, if and when that occurs
in future.
Dr. Anand Jaiswal
Senior Director - Respiratory and Sleep Medicine
Medanta - Gurugram
Dr. Bornali Dutta
Director - Pulmonary Medicine
Respiratory and Sleep Medicine
Medanta - Gurugram
Dr. Ashish Kumar Prakash
Senior Consultant - Respiratory and Sleep Medicine
Medanta - Gurugram
Use of Extracorporeal CO2 Removal to Manage Acute Severe Asthma
Management of acute severe asthma involves use of inhaled beta-2, anticholinergic agents, corticosteroids – oral or intravenous – and the use of magnesium sulphate (MgSO4). Use of non-invasive ventilation (NIV) in acute severe asthma, called asthmaticus, is not well proven. However, NIV can be tried in patients who are conscious, oriented, haemodynamically stable and not at risk of impending respiratory failure.
The need for invasive mechanical ventilation arises in the event of worsening oxygenation, respiratory distress, fatigue, worsening pH, and rising partial pressure of carbon dioxide (pCO2). Rescue therapies for refractory status asthmaticus, include heliox, inhalation of general anaesthetic agents, ketamine infusion, methyl xanthines and extracorporeal life support. Ted Kolobow and Team were the first to demonstrate the use of extracorporeal CO2 removal (ECCO2R) devices in the year 1977.
ECCO2R is a technique to decarboxylate blood and thus correct hypercapnia and respiratory acidosis. It is similar to the extracorporeal membrane oxygenation (ECMO) process, but uses lower blood flow, usually less than 1,500mL/min. Therefore, this technique has little or no impact on blood oxygenation. Initially, ECCO2R was developed to treat patients with acute respiratory distress syndrome (ARDS), but because of the progressive improvement of this technique, and its use in hospitals, ECCO2R could be proposed as a therapeutic option in cases of hypercapnic respiratory insufficiency. It can either be considered during acute and severe decompensation of chronic obstructive pulmonary disease (COPD) or in ARDS for less invasive mechanical ventilation (IMV).
Case Study
A 63-year-old male patient with a medical history of hypertension and asthma for 20 years presented in the Emergency of Medanta - Patna. He had been experiencing breathing difficulty for 2 days and his condition had significantly worsened over the past few hours.
His at-home regimen included anti-hypertensive medicines and albuterol metered dose
inhaler (MDI) as indicated. Five years ago, the patient had been admitted for steoarthritis
of the right hip and had undergone total hip replacement (THR). During the course of the treatment, he was intubated. In view of difficulty weaning, the patient was tracheostomised and had to endure a prolonged stay in the ICU. Medical records were suggestive of severe bronchospasm.
At Medanta - Patna, the patient was evaluated by the emergency team. The physical examination was notable for diffuse bilateral expiratory wheeze; lab investigations revealed increased white blood cells (WBC); chest X-ray was suggestive of hyperinflation. However, there was no focal opacity, pneumothorax or pulmonary edema. His 2D ECHO was suggestive of mild concentric left ventricular hypertrophy (LVH), no regional wall motion abnormalities (RWMA), left ventricular ejection fraction (LVEF) of 55%, grade-1 diastolic dysfunction (DD) and pulmonary arterial systolic pressure (PASP) of 18mmHg. Arterial blood gas (ABG) analysis was suggestive of type-2 respiratory failure with pH7.17, pCO2 72mmHg, pO2 95mmHg, HCO3 25.9mmol/L.
Patient was started on NIV, nebulisation with beta-2 agonist/anticholinergics and IV hydrocortisone dosage of 100mg every 6 hours. He was then shifted to the ICU. ABG at the time was pH7.17, pCO2 68.8mmHg, pO2 115mmHg, HCO3 25mmol/L.
In view of persisting acidosis and poor compliance to NIV, the patient was intubated with
endotracheal tube (ETT) of 8.5mm and started on invasive mechanical ventilation. He was administered a slow intravenous dose of 2gm MgSO4 over 1 minute. Worsening acidosis and elevated airway pressure prompted the use of intravenous Fentanyl. Despite conventional treatment (bronchodilators, steroid, MgSO4), patient continued to experience severe bronchospasm.
On Day 2 of ICU admission, ABG was pH7.098, pCO2 80.4mmHg, pO2 105.3mmHg, HCO3
21.4 mmol/L. On Day 3, he was started on inhalational sevoflurane dose of 2.2%-4% using anaesthesia machine. However, after 4 hours of use, the patient developed significant hypotension and the use of sevoflurane was stopped. Day 3 ABG was pH7.150, pCO2 75.8mmHg, pO2 90.7mmHg, HCO3 27.9mmol/L.
On Day 4, ABG stood at pH7.09, pCO2 79.4mmHg, pO2 58.0mmHg, HCO3 24.0mmol/L. In view of the patient’s condition, it was decided to try extracorporeal carbon dioxide removal. The right femoral vein was cannulated using the 11.5 French double-lumen dialysis catheter. The assembly consisted of the QUADROX-i paediatric oxygenator, Fresenius 4008S dialysis machine, air-oxygen blender and 3/16inch polyvinyl chloride (PVC) tubing. He was administered 5000 units of unfractioned heparin. Blood flow was set at 350ml/min with sweep gas flow of 5L/min to start with and was titrated according to the ABG. Patient was started on heparin infusion and dose was titrated according to activated clotting time (ACT).
Day 5 ABG of pH7.168, pCO2 55.4mmHg, pO2 107.2mmHg, HCO3 19.7mmol/L was suggestive of slight improvement. On Day 6, ABG further improved to pH7.15, pCO2 58.7mmHg, pO2 99.6mmHg, HCO3 20.0mmol/L. Considering this, ECCO2R was stopped 72 hours after its initiation. On Day 7, as the ABG parameters stood at pH7.241, pCO2 49.5mmHg, pO2 119.8mmHg, HCO3 20.8mmol/L, the patient was given pressure support trial, but he was not able to tolerate it and was continued on controlled ventilation. On Day 8, percutaneous tracheostomy was done with Portex 7.5mm tubing under bronchoscopic guidance. Procedure was uneventful and he was assessed for weaning on a daily basis. Physiotherapy, both chest and limb, was done regularly twice a day. On Day 12, the patient was decannulated and shifted to the ward. Patient was discharged on Day 15 of hospital admission.
Conclusion
Evidence for the use of ECCO2R in acute severe asthma is limited. ECCO2R may, however, be considered in the context of severe bronchospasm refractory to maximum medical therapy.
Dr. Kishore Jhunjhunwala
Director - Critical Care Medicine
Institute of Critical Care and Anaesthesiology
Medanta – Patna
Dr. Gunjan Kumar
Consultant - Institute of Critical Care and Anaesthesiology
Medanta – Patna
Chylothorax: A Rare Presentation of Tuberculosis
A 13-year-old boy presented to Medanta - Gurugram with the history of fever and loss of appetite for 2 months along with cough and fast breathing for 1 week. He also had history of significant weight loss of around 10kg in the last 2 months. There was no contributory past and/or family history. At the time of presentation, the child was alert, sick looking with tachycardia – heart rate of 136 beats/minute, tachypnea – respiratory rate of 65 breaths/minute, subcostal, intercostal retractions, and nasal flaring with room air saturation of 88%. Examination revealed markedly reduced air entry on the right side and basal bilateral crepitations. Rest of the systemic examination was within normal limits.
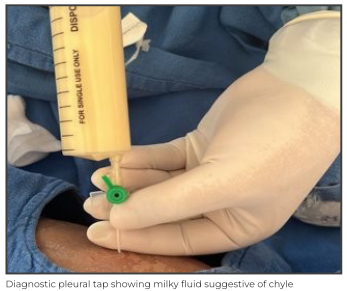
In view of the features of respiratory distress and increased work of breathing, he was immediately put on non-invasive ventilation with pressure support of 14cm of water and positive end-expiratory pressure (PEEP) of 7cm of water along with other supportive care. Chest X-ray showed right-sided consolidation with pleural effusion. In view of severe respiratory distress, urgent diagnostic pleural tap followed by therapeutic intercostal drain (ICD) was planned. Pleural tap revealed milky white fluid suggestive of chyle, which was sent for investigation. ICD drained about 700ml milky fluid, which was partly replaced with 5% albumin over slow infusion. Child clinically improved after the ICD drainage. Pleural fluid analysis showed 3500 cells with lymphocytic predominance (96%), high triglyceride levels (1815mg/dL) and chylomicron, confirming the fluid to be chyle and differentiating it from the other causes such as pseudochylothorax.
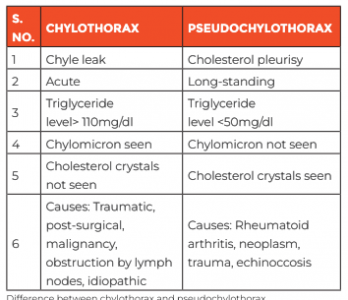
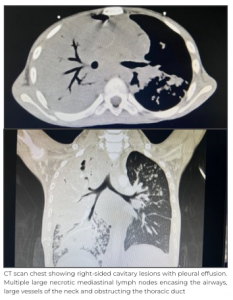
However, gene-xpert test for tuberculosis in pleural fluid came negative. Other laboratory investigations showed mild anaemia with leukocytosis. CT chest showed cavitary lesions highly suggestive of infective lesion. Provisional diagnosis of chylothorax secondary to trauma, post-surgical bacterial infection, tuberculosis infection, malignancy, immunodeficiency or pseudochylothorax was kept. Child was started on broad spectrum antibiotics and work-up for the above-mentioned differentials were sent. There was no history of trauma or surgery present, and since pleural fluid gene-xpert test result was negative, induced sputum for the same was sent for tuberculosis infection; it came back positive and was Rifampicin-sensitive. Meanwhile, the child improved clinically, and was taken off oxygen support within 48 hours of admission. ICD was removed on Day 3 without any further collection. Bacterial culture sent from the blood as well as from pleural fluid came sterile.
Final diagnosis of chylothorax secondary to new onset microbiologically confirmed Rifampicin- sensitive Pulmonary Tuberculosis. First-line anti-tubercular drugs were started along with pyridoxin. Child is being followed up in the out-patient department and is doing well with good weight gain.
Discussion
Typical characteristic of tubercular pleural effusion is that of straw-coloured fluid. This fluid is secondary to the inflammatory pleural reaction to tubercular bacilli. Tubercular effusion presenting as chylothorax is rare. There are a few, isolated case reports of this phenomenon. Normal lymph production ranges from 1.5 to 2.5 liters per day, which drains into the venous system. Any obstruction to this drainage, therefore, leads to high-volume collection in the related cavity (pleura/peritoneum). Pathogenesis of chylothorax is related to obstruction or disruption of the thoracic duct by inflamed and necrotic lymph nodes leading to leakage of the chyle into the pleural space. Gross characteristics are that of a milky fluid due to high triglyceride content. Lymph is inherently bacteriostatic and non irritant.
Hence, collections rarely lead to high fever or pain. Most symptoms are likely due to the sheer large volume and associated pressure effects.
Treatment is most frequently conservative, aiming for the resolution of the primary cause. Continuous loss of T cells, fat-soluble vitamins and proteins may lead to malnutrition and infections. Additionally, drainage of the accumulated chyle may also be required to relieve pressure effects as it was in this case. Low-fat or fat-free diet with medium chain triglycerides can be helpful in the dietary management. Rarely, octreotide – a new somatostatin analogue – can be administered in severe presentations to reduce the chyle
production. Interventional radiology guided embolisations of the thoracic duct is considered in refractory cases.
Clinicians must be aware of this rare presentation of tuberculosis and while analysing the chylous pleural effusion, tuberculous chylothorax disease must be considered as one of the possibilities. All possible efforts should be made to look for the microbiological confirmation of tuberculosis before making the final diagnosis. Prompt anticipation with early diagnosis is crucial in the outcome as tuberculous chylothorax is a completely
curable disease.
Dr. Rajiv Uttam
Director and HOD - Paediatrics,
PICU and Paediatric Emergency
Institute of Women and Children
Medanta – Gurugram
Dr. Praveen Khilnani
Chairman - Paediatrics, Paediatric Pulmonology and Paediatric Critical Care Institute of Women and Children
Medanta – Gurugram
Dr. Mukul Pandey
Consultant - Paediatrics, PICU
and Paediatric Emergency
Institute of Women and Children
Medanta – Gurugram
Spotlight
Medanta Launches New Facility at DLF Cyber Park in Gurugram
Medanta has launched its latest facility at DLF Cyber Park in Gurugram with a view to help working professionals take care of their health despite significant professional and personal commitments. Mr. Pankaj Sahni (Group CEO, Medanta) and Dr. Sushila Kataria (Senior Director, Internal Medicine, Medanta - Gurugram) inaugurated the centre, which offers consultation with Medanta experts, Medicine Delivery and Sample Collection services to various establishments operating out of the office complex.



It has a fully stocked pharmacy that ensures delivery of high-quality medication and healthcare products to the work desk.
Its conveniently located sample collection centre does swift, hygienic sample pick-ups in adherence with all protocol. These services are backed by Medanta expert doctors and nutritionists who ensure that working professionals never have to compromise with their health.
Medanta has always been at the forefront of delivering advanced medical solutions catering to the diverse needs of people. The establishment of this new facility is an initiative to deliver our comprehensive healthcare services to the corporate community.
We see this as a significant stride in our mission to bring world class healthcare services closer to people.
 Dr. Shandip Kumar Sinha
Dr. Shandip Kumar Sinha
Director- Paediatric Surgery
and Paediatric Urology
Medanta - Gurugram
Paediatric surgeon with over 15 years of experience and expertise in performing minimally invasive procedures in newborns and small children. He also has expertise in paediatric urology, including management of antenatally detected anomalies.
 Dr. Praney Gupta
Dr. Praney Gupta
Senior Consultant - Paediatric Surgery
and Paediatric Urology
Medanta – Gurugram
Paediatric surgeon with expertise in performing laparoscopic gastrointestinal surgeries, urological surgeries, including cystourethroscopy, thoracic surgery, congenital and neonatal surgeries in addition to emergency surgeries.
 Dr. Yogesh Kumar Arya
Dr. Yogesh Kumar Arya
Consultant - Paediatrics, PICU and Paediatric Emergency
Medanta - Gurugram
Paediatrician with expertise in paediatric critical care medicine and managing children with infectious diseases and emergencies.
 Dr. Hari Prasad Achanti
Dr. Hari Prasad Achanti
Associate Consultant - Surgical Oncology
Medanta - Patna
Surgical oncologist with expertise in complex gastrointestinal oncology procedures, head and neck surgeries with complex reconstruction requirements, fertility and function-preserving gynaecological procedures in addition to breast oncology and breast preservation surgeries.
 Dr. Neha Moreshwar Mahajan
Dr. Neha Moreshwar Mahajan
Associate Consultant - Obstetrics
and Gynaecology
Medanta - Gurugram
Gynaecologist with expertise in managing high-risk pregnancy and delivery, complex gynaecological surgeries in addition to adolescent health and perimenopausal health issues.
 Dr. Ankur Anand
Dr. Ankur Anand
Associate Consultant - Neurosurgery
Medanta - Patna
Neurosurgeon with expertise in neurotrauma (brain and spine injury), minimally invasive brain and spine surgery, brain tumour and endoscopic spine surgery.
 Dr. Sailen Kumar Bana
Dr. Sailen Kumar Bana
Associate Consultant - Paediatric Gastroenterology and Hepatology
Medanta - Gurugram
Paediatric gastroenterologist with expertise in paediatric and adolescent gastroenterology and hepatology. He specialises in endoscopy, and in the treatment of pancreatitis, irritable bowel syndrome in addition to nutritional issues in children and adolescents.
 Dr. Ankit Jain
Dr. Ankit Jain
Associate Consultant - Neonatology
Medanta - Lucknow
Neonatologist with expertise in managing Level-3 NICU, including ventilation, complex neonatal procedures and high-risk developmental follow-up of newborns discharged from NICU.