THE EXCHANGE | Newsletter June 2019

Synchronous Malignancy
Introduction
Synchronous multiple primary malignancies in the female genital tract are infrequent. 50 - 70% of them correspond to synchronous cancers of the endometrium and ovary. Synchronous primary tumours account for 0.7 - 1.8% of all gynaecological tumours. Presence of multiple cancers in a single patient was first reported more than 100 years ago.
It was first described by Billroth in 1879. The neoplasm may be limited to a single organ or involve multiple and anatomically separate organs. Double malignancies are most common in the female reproductive tract, followed by head and neck malignancies.
Multiple primary tumours are divided into two categories. Two cancers are identified/detected at the same time (synchronous), or one may follow the other after a period of two to six months (metachronous). Synchronous tumours occur uncommonly with the most common site for synchronously existing multiple tumours being the breast.
To diagnose synchronous malignancy is a challenge, and hence diagnostic criteria which have to be fulfilled for synchronous malignancy are that each tumour should be histologically distinct, and the possibility that one is a metastasis of the other must be excluded.
he pathophysiology behind the occurrence of multiple primary malignancies has been theorized to be common carcinogen induced multiple cancers in an exposed epithelial surface, called field-cancerization, late side effects of treatment used to treat another tumor in the past, a genetic predisposition to neoplasia. Other possible factors are persistent carcinogen exposure from environment, progressive ozone depletion and effects of ionizing radiation.
The co-existence of breast and ovarian cancers in the same individual should raise suspicion of a hereditary process. Patients with either BRCA1 or BRCA2 germ-line mutations have an average risk of 39% and 11% respectively of developing ovarian cancer by the age of 70; they have a risk of 35 - 85% of developing breast cancer in their lifetime.
We here report pathologic features in a BRCA2 germ-line mutation diagnosed with synchronous breast and ovarian cancers.
Case study
A 58-year-old menopausal female came to Medanta - The Medicity with a history of constipation and abdominal distension for three months. She had comorbidities of hypertension, hypothyroidism, chronic kidney disease and atrial fibrillation for which she had a pacemaker in situ. She had no family history of cancer.
CECT whole abdomen showed a heterogeneous mass lesion measuring 11.7x11.8x8.9 cm in the pelvis, closely abutting the posterior surface of the uterus, moderate ascites, omental thickening and fat stranding. CA-125 was 1000 U/ml. Upper GI endoscopy and colonoscopy were normal. Biopsy from adnexal mass done at another centre indicated high-grade serous carcinoma of ovary.
Introduction
Synchronous multiple primary malignancies in the female genital tract are infrequent. 50 - 70% of them correspond to synchronous cancers of the endometrium and ovary. Synchronous primary tumours account for 0.7 - 1.8% of all gynaecological tumours. Presence of multiple cancers in a single patient was first reported more than 100 years ago.
It was first described by Billroth in 1879. The neoplasm may be limited to a single organ or involve multiple and anatomically separate organs. Double malignancies are most common in the female reproductive tract, followed by head and neck malignancies.
Multiple primary tumours are divided into two categories. Two cancers are identified/detected at the same time (synchronous), or one may follow the other after a period of two to six months (metachronous). Synchronous tumours occur uncommonly with the most common site for synchronously existing multiple tumours being the breast.
The pathophysiology behind the occurrence of multiple primary malignancies has been theorized to be common carcinogen induced multiple cancers in an exposed epithelial surface, called field-cancerization, late side effects of treatment used to treat another tumour in the past, a genetic predisposition to neoplasia. Other possible factors are persistent carcinogen exposure from environment, progressive ozone depletion and effects of ionizing radiation.
The co-existence of breast and ovarian cancers in the same individual should raise suspicion of a hereditary process. Patients with either BRCA1 or BRCA2 germ-line mutations have an average risk of 39% and 11% respectively of developing ovarian cancer by the age of 70; they have a risk of 35 - 85% of developing breast cancer in their lifetime.
We here report pathologic features in a BRCA2 germ-line mutation diagnosed with synchronous breast and ovarian cancers.
Case study
A 58-year-old menopausal female came to Medanta - The Medicity with a history of constipation and abdominal distension for three months. She had comorbidities of hypertension, hypothyroidism, chronic kidney disease and atrial fibrillation for which she had a pacemaker in situ. She had no family history of cancer.
CECT whole abdomen showed a heterogeneous mass lesion measuring 11.7x11.8x8.9 cm in the pelvis, closely abutting the posterior surface of the uterus, moderate ascites, omental thickening and fat stranding. CA-125 was 1000 U/ml. Upper GI endoscopy and colonoscopy were normal. Biopsy from adnexal mass done at another centre indicated high-grade serous carcinoma of ovary.
PET scan performed at Medanta revealed hypermetabolic heterogeneously enhancing mitotic pelvic mass, likely originating from the right ovary, inseparable from the uterus with the right ovary not separately visualized. Moderate ascites with multiple omento-peritoneal metastases and moderate left pleural effusion. Hypermetabolic heterogeneously enhancing nodular lesion highly suspicious of a synchronous breast primary was noted in the left breast.
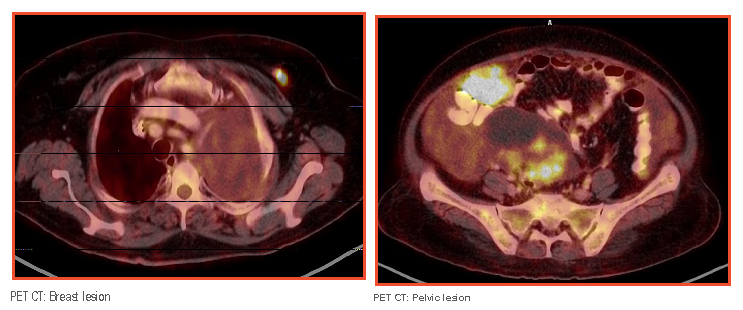
Image guided biopsy from omentum was highly indicative of metastatic deposits of a serous carcinoma. Pleural fluid cytology and cell block turned out to be negative for malignant cells. Breast biopsy indicated a malignant invasive ductal carcinoma, grade-3, ER/PR/HER 2neu negative.
The patient was planned for primarily surgical management after complete preoperative workup, and tumour board discussion.
Diagnostic laparoscopy followed by exploratory laparotomy, removal of bilateral ovarian masses, both fallopian tubes along with the removal of the uterus, total omentectomy and removal of visible deposits was done. The mass was densely adherent to the recto-sigmoid surface. During removal of the same, there was a tear in the recto-sigmoid for which repair was done and prophylactically, a diversion loop transverse colostomy was conducted. Optimal cyto reduction was achieved, and there was no residual disease. In the same sitting, breast surgeons performed left wire guided wide local excision of breast mass with sentinel lymph node biopsy. The histopathology was stage-3 serous carcinoma of ovary and invasive ductal carcinoma breast.
In view of the separate synchronous primaries in the ovary and breast, the patient was recommended molecular studies for BRCA1 and 2. She is reported to be BRCA2 positive.
The patient had stage III high-grade serous carcinoma of the ovary for which she required chemotherapy. She was referred to a medical oncologist and planned for 18 weeks of dose dense chemotherapy in view of multiple co-morbid conditions. Chemotherapy was to be followed by radiation therapy for IDC, left breast. The patient was not tolerating chemotherapy well, and her leucocytes and platelet count remained low. Initially, she could manage to complete nine cycles.
In the PET CT done after nine cycles, post-operative changes were seen in the left breast and abdomen. No abnormal FDG-avid residual/recurrent lesion in the left breast was noticed. But there was a subcentimetric rounded suspicious node in left axilla and some avidity in the vaginal vault which needed further evaluation. Upon evaluation, the suspicious node in the breast was found to be non-malignant, and the vault lesion which is avid is being closely watched. The patient resumed her chemotherapy after a month’s break and has completed the 16th cycle till now. She is under periodic follow-up and is due for the 17th cycle.
This case brings forth the importance of detailed clinical evaluation and multi-disciplinary approach for the management of cancer.
https://www.medanta.org/dr-sabhyata-gupta/
Case of Coronary Mycotic Aneurysm - Cascade of Complications
65-year-old male, diabetic and hypertensive, had experienced chest pain in July, 2017. He was taken to a local hospital, where he was diagnosed with non-ST-segment-elevation acute coronary syndrome (NSTEACS). He underwent coronary angiography (CAG) which revealed double vessel disease. PTCA + Stent*(2) was done to culprit artery (LCX and OM). The procedure went uneventful and the patient was discharged in satisfactory condition.
Three months later, the patient developed refractory angina on exertion. Hence CAG was repeated in November, 2017 (at the same hospital) which revealed stent thrombosis/early in stent restenosis. Another PTCA + stent was therefore done to LCX-OM.
Since then the patient persistently remained unwell as he was getting intermittent spikes of fever, lethargy and chest pain. He presented at Medanta - The Medicity with these issues. Initial investigations revealed raised inflammatory markers and leukocytosis. Echo showed LVEF-45, moderate MR, mild pericardial effusion, no tamponade. Differentials kept in mind at that point of time were:
- Dressler Syndrome
- Pneumonia
- Some recent infection + Angina
Infection work-up including cultures was sent. Transesophageal echocardiography (TEE) was done to rule out infective endocarditis and assess the severity of mitral regurgitation. CAG was performed to rule out stent thrombosis, but findings of angiogram presented a completely different picture. There were two large aneurysms around the site of implanted stents in left circumflex and obtuse marginal arteries. Possibly aneurysms were mycotic and were giving an impression of impending rupture, which could have been fatal. Infection Disease (ID) and Cardiothoracic and Vascular Surgery (CTVS) teams were immediately involved and after clearances and under antibiotic covers, the patient underwent LCX plication plus SVG to LAD. All subsequent cultures came negative. His post-operative period remained fairly uneventful and he was discharged in stable condition with appropriate antibiotic therapy. He was advised to follow up with ID team along with cardiology and CTVS.
Having experienced such complication and suffering in such a short span, followed by prompt follow up procedure, the issue was far from over. Following 20 days of discharge, the patient landed in our Emergency Department complaining of a non-healing sternal wound. He was diagnosed with osteomyelitis and underwent surgical debridement by the Plastic Surgery team. All cultures were once again negative. He was closely followed by the ID team as well. The patient got discharged after the surgery, on IV antibiotics.
A month later, the patient once again returned to our Emergency Department, this time with fever and worsening wound condition. He had to undergo repeat surgical debridement and another course of prolonged antibiotics. Cultures remained negative.
The patient did fairly well over the course of next one year, barring persistent low grade fever, for which he was started on empirical ATT outside.
He presented again in our Cardiology OPD in March, 2019, with complaints of cough, chest pain and intermittent low grade fever. A new finding was revealed this time by CAG and supported by CT aortogram. The CAG suggested native DVD and a pseudoaneurysm in the ascending aorta and we could not find the SVG to LAD graft. The CT revealed a pseudoaneurysm at the site of graft with no distal flow.
The possibility of a repeat surgery and ongoing infection was discussed in detail between the CTVS and ID teams negotiating further options. Repeat surgery was refused by the surgeons because of the high risk involved. Since the cultures were not positive until now, and the way infection was behaving, the ID team suspected non tubercular mycobacterium or less virulent staphylococcus being the culprit bug. As surgical biopsy was not feasible, the patient was initiated on empirical therapy for these infections. The fever was now better and the patient was improving. Next piece of the puzzle was pseudoaneurysm in the ascending aorta, a constant threat to his life.
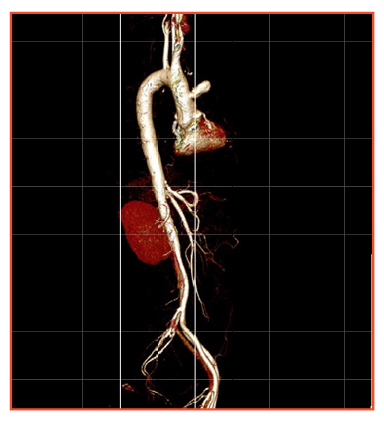
A PDA device was used to plug the orifice of pseudoaneurysm, retrogradely via the femoral artery access. Patient tolerated the procedure well and was discharged in a stable condition. At three months of follow up, the patient continues to be asymptomatic, without fever, and leading an active life.

This was an unfamiliar clinical scenario for which there was no detailed literature. Treatment possibilities were endless. We stuck to our endeavour of bailing the patient out with innovative thinking and meticulous execution with which we were able to salvage the patient.