
THE EXCHANGE | Newsletter May 2021
Medanta Initiates Monoclonal Antibody Therapy for COVID-19 Treatment
Local resident of Lucknow receives first Monoclonal Antibody Therapy in UP at Medanta - Lucknow
84-year-old Mr. Mohabbat Singh receives first Monoclonal Antibody Therapy in Medanta - Gurugram
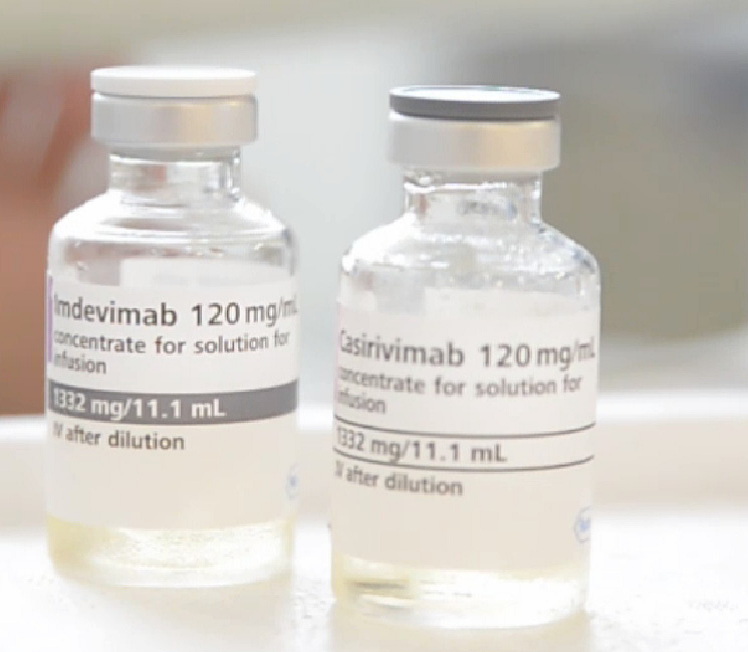
Recently approved by the Drugs Controller General of India (DCGI), Medanta started administering monoclonal antibody therapy to COVID-19 positive patients. The antibody combination of Casirivimab and Indevimab is a cutting-edge treatment that will provide protection to COVID-19 positive patients with mild or moderate symptoms before they deteriorate further or require hospitalization. Delivered through a single infusion intravenously or subcutaneously, the treatment can be provided on an outpatient basis and marks a dramatic shift in COVID-19 care in India.
The monoclonal antibody therapy is most suited for high-risk COVID-19 patients who are within first ten days of symptom onset and meet any of the following criteria:
- Age 65 years or older
- Obesity with BMI>35
- Type 1 or type 2 diabetes mellitus
- Chronic kidney disease, including those on dialysis
- Chronic liver disease
- Currently receiving immunosuppressive treatment
- Age>55 having either heart disease, or hypertension, or chronic lung disease
High risk patients between 12 – 17 years of age may also be eligible if they have any of the following conditions:
- BMI ≥85th percentile for their age and gender based on CDC growth charts
- Sickle cell disease, or congenital or acquired heart disease
- Neurodevelopmental disorders (e.g., cerebral palsy)
- Medical-related technological dependence, for example, tracheostomy, gastrostomy, or positive pressure ventilation (not related to COVID-19), asthma, reactive airway or other chronic respiratory disease that requires daily medication for control
The therapy is most effective when administered within the first ten days of symptom onset, and is not recommended in patients who:
- are hospitalized due to severe COVID-19, or
- require oxygen therapy due to COVID-19, or
- require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-Covid-19 related comorbidity.
Patients treated with Casirivimab and Imdevimab should continue to self-isolate and follow all infection control measures.
Monoclonal antibody therapy is a fast, effective treatment for COVID -19. It will save patients at highest risk from falling critically ill, getting hospitalized or possibly dying of complications from COVID-19. We are glad the treatment is finally available in India and, we look forward to serving our patients with this therapy and saving more lives.

Dr. Naresh Trehan
Chairman and Managing Director - Medanta
Monoclonal antibody therapy is a fast, effective treatment for COVID -19. It will save patients at highest risk from falling critically ill, getting hospitalized or possibly dying of complications from COVID-19. We are glad the treatment is finally available in India and, we look forward to serving our patients with this therapy and saving more lives.
Brain-Pacemaker Surgery Gives New Lease of Life
When 44-year-old Mr. Pandey visited Medanta Gurugram in February 2021, he had been suffering from severe tremors (shaking movements), rigidity (whole-body stiffness) and bradykinesia (slowness of movements) as a result of intractable Parkinson’s disease for several years. He had initially responded to oral medications. But over the last few years, the effect of medication had reduced and the patient started experiencing dyskinesias as side effect due to escalating dosage of medications. The medication ‘on-time’ (active time) had come down to only one-and-a-half hours. The patient had limited mobility and was mostly confined to home due to advanced Parkinson’s Disease. His quality of life was severely impaired.
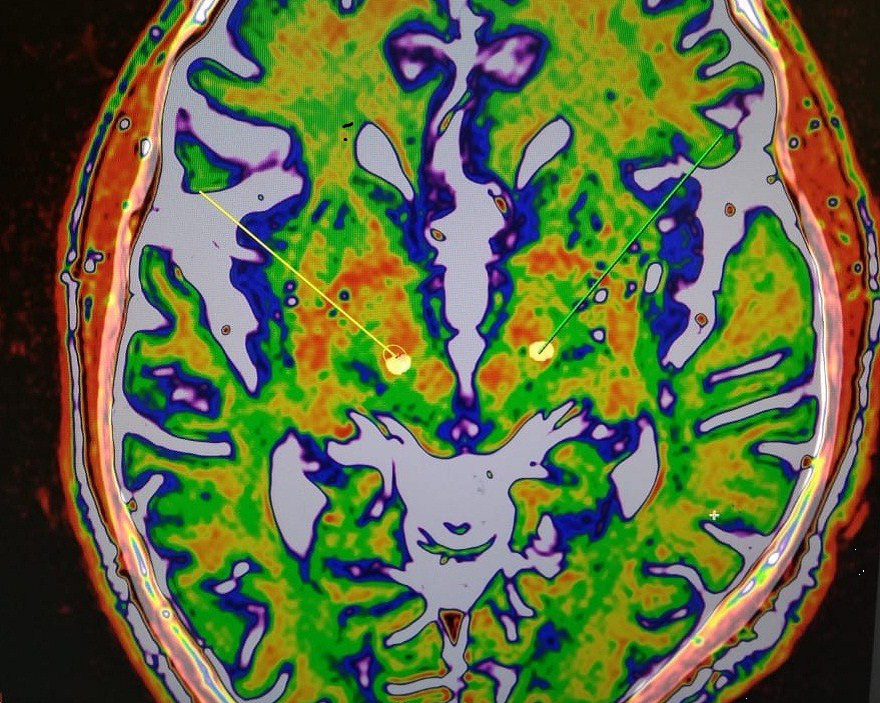
Perfect overlapping between the before-surgery brain images (target planning images) and the after-surgery (post brain-electrodes implantation) images, indicating precise placements of the brain-pacemaker electrodes.
Patient was evaluated by the Medanta Institute of Neurosciences Movement Disorder Clinic, whereby he was advised for Deep Brain Stimulation (Sub-thalamic Nucleus Stimulation) for advanced Parkinson’s disease.
Stereotactic Brain CT/MR fusion was done to locate the bilateral sub-thalamic nucleus, intra-operative MER recordings and stimulation were done to ensure the best trajectory for chronic stimulation. Patient was eventually implanted with the constant-voltage based Brain-Pacemaker (Deep Brain Stimulation) System.
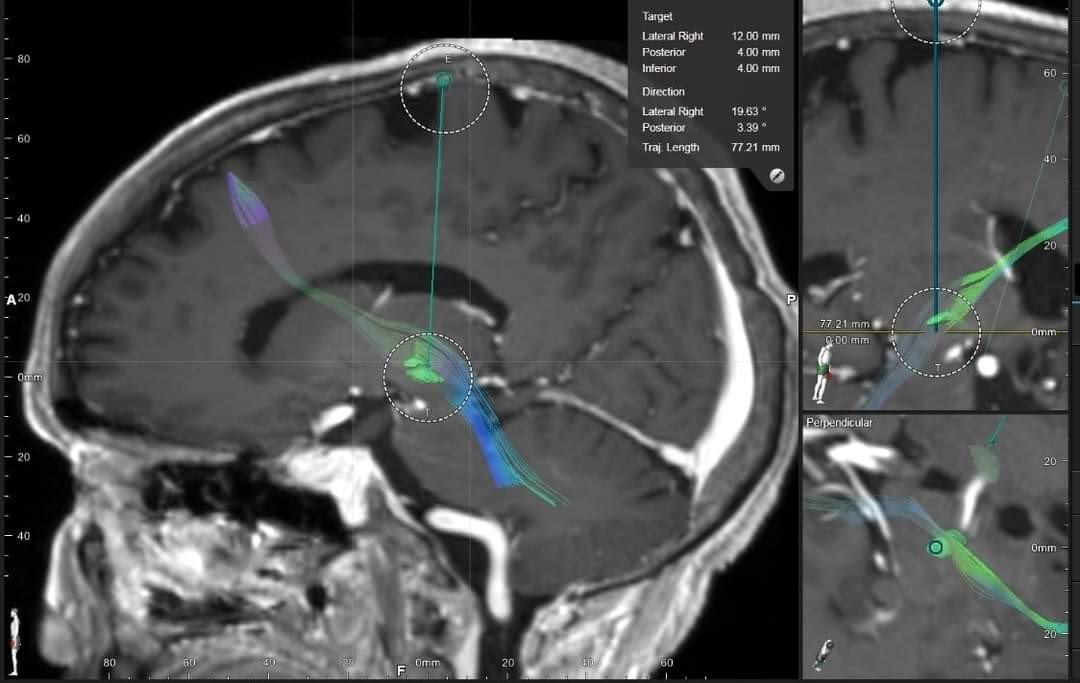
Tractography-aided technology used in this patient’s brain-pacemaker surgery is the future of deep brain stimulation surgery whereby entire brain-circuits dealing with specific brain-functions (connectomic surgery) can be modulated rather than just stimulating static brain targets (as is conventionally done).
Considerable improvement in patient’s symptoms was noted during the course of this awake neurosurgical procedure itself. The brain-pacemaker was then programmed and checked for best parameters.
In his recent follow-up, the patient was significantly mobile with steadily improving ‘On-time’; he was doing all his activities independently. Medication dosage has also reduced considerably and so have the side-effects (dyskinesias). The patient’s quality of life has drastically improved.
Tractography-aided technology used in this patient’s brain-pacemaker surgery is the future of deep brain stimulation surgery whereby entire brain-circuits dealing with specific brain-functions (connectomic surgery) can be modulated rather than just stimulating static brain targets (as is conventionally done).
Perfect overlapping between the before-surgery brain images (target planning images) and the after-surgery (post brain-electrodes implantation) images, indicating precise placements of the brain-pacemaker electrodes.
Deep Brain Stimulation
Deep brain stimulation (DBS) is a surgery to implant a device that sends electrical signals to brain areas responsible for body movement. Electrodes are placed deep in the brain and are connected to a stimulator device. Similar to a heart pacemaker, a neurostimulator uses electric pulses to regulate brain activity. DBS can help reduce the symptoms of tremor, slowness, stiffness, and walking problems caused by Parkinson’s disease, dystonia, or essential tremor. Successful DBS allows people to potentially reduce their medications and improve their quality of life.
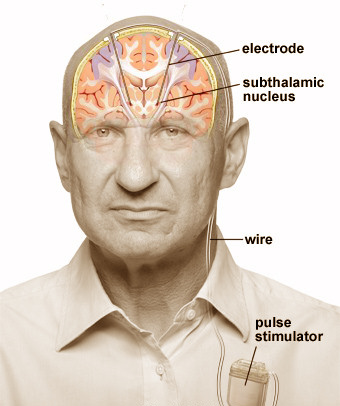
Overview of a deep brain stimulator (DBS). Electrodes are placed deep within the brain through small holes in the skull. The electrodes are connected by an extension wire to a battery-powered stimulator placed under the skin of the chest. Because the left side of the brain controls the right side of the body and vice versa, DBS is commonly performed on both sides of the brain.
In deep brain stimulation, electrodes are placed in a specific area of the brain depending on the symptoms being treated. The electrodes are placed on both the left and right sides of the brain through small holes made at the top of the skull. The electrodes are connected by long wires that travel under the skin and down the neck to a battery-powered stimulator under the skin of the chest. When turned on, the stimulator sends electrical pulses to block the faulty nerve signals causing tremors, rigidity, and other symptoms.
The doctor programs the stimulator settings with a wireless device at periodic intervals. The stimulation settings can be adjusted as a patient’s condition changes over time. Unlike other surgeries, such as pallidotomy or thalamotomy, DBS does not damage the brain tissue permanently.
DBS is very effective at reducing dyskinesias, the uncontrolled wiggling movements caused by high doses of levadopa medication. Typically, DBS will help make your symptoms less severe so that lower medication doses may be used.
A patient may be a candidate for DBS if they have:
- A movement disorder with worsening symptoms (tremor, stiffness) and their medications have begun to lose effectiveness.
- Troubling “off” periods when their medication wears off before the next dose can be taken.
- Troubling “on” periods when they develop medication-induced dyskinesias (excessive wiggling of the torso, head, and/or limbs).
DBS can help treat many of the symptoms caused by:
- Parkinson’s disease Tremor, rigidity, and slowness of movement caused by the death of dopamine-producing nerve cells responsible for relaying messages that control body movement.
- Essential tremor Involuntary rhythmic tremors of the hands and arms, occurring both at rest and during purposeful movement. Also may affect the head in a “no-no” motion.
- Dystonia Involuntary movements and prolonged muscle contraction, resulting in twisting or writhing body motions, tremor, and abnormal posture. May involve the entire body, or only an isolated area. Spasms can often be suppressed by “sensory tricks,” such as touching the face, eyebrows, or hands.
Successful DBS is attributable to:
- Appropriate patient selection
- Appropriate selection of the brain area for stimulation,
- Precise positioning of the electrode during surgery, and
- Experienced programming and medication management
Overview of a deep brain stimulator (DBS). Electrodes are placed deep within the brain through small holes in the skull. The electrodes are connected by an extension wire to a battery-powered stimulator placed under the skin of the chest. Because the left side of the brain controls the right side of the body and vice versa, DBS is commonly performed on both sides of the brain.

